Hcn Is Mixed With Water. Which Is the Best
It partially ionizes in water solution but unionized HCN molecules do remain mixed with the water. 1 Answer anor277 Jun 10 2016 HC-Naq H_2Ol rightleftharpoons H_3O -C-Naq.
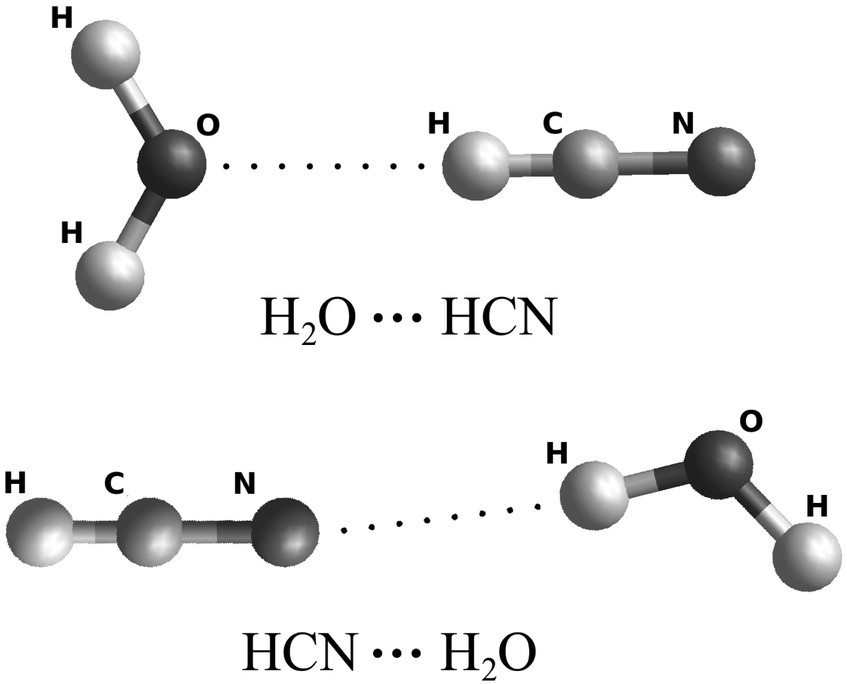
Theoretical Study Of Hcn Water Interaction Five Dimensional Potential Energy Surfaces Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C6cp07894j
HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals.
. HCN is a molecule. View more similar questions or ask a new question. Hydrocyanic acid is a liquid of hydrogen cyanide in water.
HCN is a molecule. Net ionic equation for solid sodium cyanide added to. The chemical formula of Hydrocyanic acid is HCN.
A 500 mL sample of 0436 M NH4NO3 is diluted with water to a total volume of 2500 mL. It is usually sold commercially as an aqueous solution containing 2 to 10 hydrogen cyanideThe aqueous solutions of hydrogen cyanide decomposes. It partially ionizes in water solution but unionized HCN molecules do remain mixed with the water.
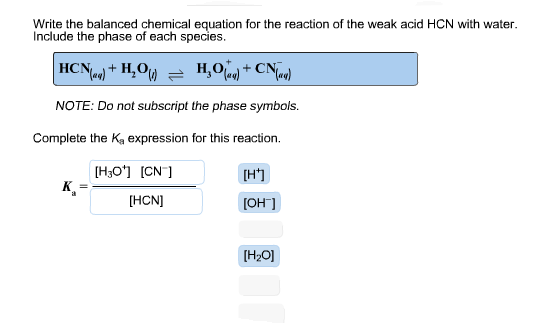
How do you complete the Ka expression for this reaction. Ka for HCN 62 x 10. Rank the pHs of each of the solutions when each are titrated to the equivalence point from highest to lowest pH.
Methane CH4 ammonia NH3 and oxygen O2 can react to form hydrogen cyanide HCN and water according to this equation. Write the balanced chemical equation for the reaction of the weak acid HCN with water. It is a colorless extremely poisonous and flammable liquid that boils slightly above room temperature at 256 C.
CH4 NH3 O2 HCN H2O You have 8 g of methane and 10 g of ammonia in excess oxygen. Hydrocyanic acid is a colorless liquid whose vapor is lighter than air and dissipates rapidly. Include the phase of each species.
Hydrogen cyanide sometimes called prussic acid is a chemical compound with the chemical formula HCN. You titrate each with 0100 M NaOH aq. Solutions of each of the following acids.
Chemistry Chemical Reactions Chemical Equations. What is the ammonium nitrate concentration in the resulting solution. HCN HF HCl and HC2H3O2.

Solved Label Each Reactant And Product In This Reaction As A Chegg Com
10 Chemical Reactions Leading To Formation Of Hydrocyanic Acid Hcn Download Scientific Diagram

Comments
Post a Comment